TRICS-IV Trial
-
Principal Investigator

Professor David Scott
Project Details

TRICS IV: Transfusion Requirements in Younger Patients Undergoing Cardiac Surgery
An international, multi-centre, open-label, randomised controlled trial (RCT) of a restrictive versus liberal transfusion strategy in higher risk patients ≤65 years of age having cardiac surgery on cardiopulmonary bypass, using a superiority trial design.
1,440 patients will be enrolled internationally.
Hypothesis: A higher haemoglobin (Hb) concentration for red blood cell (RBC) transfusion (liberal transfusion strategy) will be superior to a restrictive strategy in terms of vital organ function (heart, brain and kidney) and mortality 6 months after cardiac surgery.
Hepcidin and Iron Storage Sub-Study: The relationship of hepcidin on patient outcomes after cardiac surgery
A sub-study of the Australian participants in the TRICS IV trial to investigate whether iron status, in particular hepcidin levels, are associated with adverse outcomes.
Major Hypothesis: Baseline hepcidin concentration is associated with clinical outcomes after cardiac surgery, when adjusted for other associated risk factors.
Minor Hypothesis: Haemoglobin concentration at 30 days is associated with clinical outcomes after cardiac surgery, when adjusted for other associated risk factors.
Current Recruitment Numbers
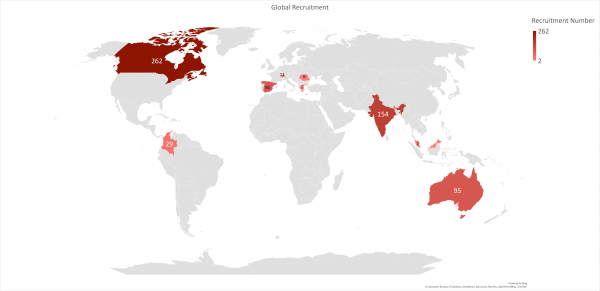 Global Recruitment Number as of 28/03/2024
Global Recruitment Number as of 28/03/2024
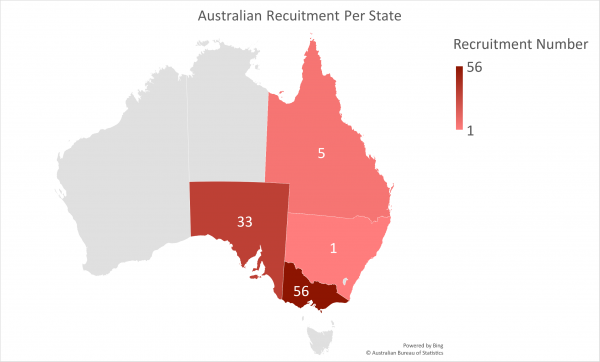
Australia Recruitment Number as of 28/03/2024
Frequently Asked Questions (27th March 2024)
Investigators
Lead Investigators (Canada)
Professor David Mazer, St. Michael’s Hospital (Toronto, ON, Canada)
Associate Professor Nadine Shehata, Mount Sinai Hospital (Toronto, ON, Canada)
Lead Investigator (Australia)
Professor David A Scott, Department of Critical Care, University of Melbourne and St Vincent's Hospital, Melbourne
Co-investigators (Australia)
Professor Alistair Royse, Dept of Surgery, University of Melbourne
Dr Raymond Hu, Dept of Critical Care, University of Melbourne; Austin Health
Professor John Fraser, University of Queensland
Professor David Mazer, St Michael’s Hospital, Toronto, Canada
Associate Professor Nadine Shehata, Mount Sinai Hospital, Toronto, Canada
Professor Paul Bannon, University of Sydney
Professor James Isbister, University of Sydney
Professor Paul Myles, Monash University
Professor Colin Royse, University of Melbourne,
Dr Reuben Slater, Dept of Critical Care, University of Melbourne; St Vincent’s Hospital, Melbourne
Dr Thomas Painter, University of Adelaide
Trial Coordinator (Australia)
Bhavita Patel, Department of Critical Care, University of Melbourne
Research funding
MRFF ICTC Grant $869,565 (2021-2024)
Collaborators
St Vincent's Hospital, Melbourne
Unity Health Toronto (Toronto, ON, Canada)
Australian and New Zealand College of Anaesthetists Clinical Trials Network (ANZCA CTN)
Publications
Murphy GJ, Pike K, Rogers CA, Wordsworth S, Stokes EA, Angelini GD, Reeves BC; TITRe2 Investigators. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med. 2015 Mar 12;372(11): 997-1008.
Mazer CD, Whitlock RP, Fergusson DA, et al. Restrictive or Liberal Red-Cell Transfusion for Cardiac Surgery. N Engl J Med. 2017; 377(22): 2133-44. 6.
Shehata N, Mistry N, da Costa BR, et al. Restrictive compared with liberal red cell transfusion strategies in cardiac surgery: a meta-analysis. Eur Heart J. 2019; 40(13): 1081-8.
Clinicaltrials.gov Identifier: NCT04754022
ANZCTR Identifier: ACTRN12621000952842 (Hepcidin and Iron Storage Sub-Study)
Twitter: #TRICS4AUS
Research Group
Anaesthesia, Perioperative and Pain Medicine
School Research Themes
Key Contact
For further information about this research, please contact the research group leader.
Department / Centre
MDHS Research library
Explore by researcher, school, project or topic.